Press Releases - 2006
Happy Holidays from Masimo!
This has been an exciting and successful year for all of us here at Masimo, and we wish to share our success with charitable organizations that you choose by making a donation to them on your behalf.
| This year, we have seen increasingly strong clinical adoption of our revolutionary Masimo SET Read-Through Motion and Low Perfusion pulse oximetry technology, with nearly 400,000
systems in use worldwide. We are especially grateful that we were able to introduce the Second Revolution in Oximetry-Masimo Rainbow SET Pulse CO-Oximetry-and that it has already been responsible for
saving lives by allowing clinicians to diagnose, treat and manage their patients' condition through the continuous and noninvasive measurement of carboxyhemoglobin, methemoglobin, oxygen saturation,
perfusion index and pulse rate.
Building on this solid foundation, we plan to introduce a number of new technologies in 2007 that continue our mission to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and new applications. As a show of gratitude to those of you who have supported the cause for better care-and to fulfill our heart-felt responsibility to give something back to some of the organizations committed to better care and a better world-Masimo would like to donate $10 to the charity of your choice. We will make this donation in the name of each person who is an official member of Livewire as of today and who responds to this Livewire with their choices from the list below:
|
 |
Please send us an e-mail at: charity@masimo.com to specify your selection. Only requests by e-mail to this address will be processed.
We also would appreciate any comments or suggestions you might have that would help us as we strive to fulfill our mission and adhere to our guiding principles.
Masimo Mission Statement:
Improving patient outcomes and reducing cost of care by taking noninvasive monitoring to new sites and applications.
Masimo Guiding Principles:
- Remain faithful to your promises and responsibilities.
- Thrive on fascination and accomplishment and not on greed and power.
- Make each day as fun as possible.
- Strive to make each year better than the year before, both personally and for the Team.
- Do what is best for patient care.
Thank You!
New Clinical Research: Masimo Rainbow SET Pulse CO-Oximetry Technology Shown Effective and Efficient in Detecting Carbon Monoxide Poisoning in Multiple Clinical Settings
New studies presented at the 2006 AARC Congress reinforce growing clinical evidence
on the efficacy of Masimo's breakthrough Rainbow SET Technology
Irvine, California December 19, 2006 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that two new independent studies and one case report were presented last week at the 2006 American Association of Respiratory Care (AARC) Congress in Las Vegas, each highlighting the significant clinical benefits to be gained by noninvasively screening patients for carbon monoxide poisoning using the "rapid," "inexpensive" and "reliable" Masimo Rainbow SET Pulse CO-Oximetry technology.
In a study led by Dr. Robert Partridge and Dr. Gregory Jay of Rhode Island Hospital at Brown University Medical School, a team of researchers performed a study to assess baseline carbon monoxide (CO) levels of nearly 5,000 patients presenting to the emergency room. To accomplish this, all pulse oximeters in the emergency department (ED) were replaced with Masimo Rainbow SET Pulse CO-Oximeters and the ED staff began assessing baseline carboxyhemoglobin (COHb) levels of all adult patients as part of the standard triage process. In addition to confirming suspected cases of CO toxicity (COT) from smoke inhalation, there were nine unsuspected cases of COT discovered, in just three months, in patients who presented with non-specific symptoms or unrelated complaints. Toxic COHb levels ranged from 16-33% and were confirmed with an invasive laboratory blood test. If this rate were indicative of all US hospitals, it would equate to as many as 50,000 cases of unsuspected CO toxicity annually.
The study concluded that the use of Masimo Rainbow SET as a noninvasive test for COT can effectively and efficiently be performed at ED triage, and that "unsuspected COT may be identified using noninvasive COHb screening and the prevalence of COT may be higher than previously recognized."
The team from Brown University also presented a case report of a previously healthy 52-year old non-smoking female who was brought to the ED complaining of nausea, headache, dizziness, and feeling cold. The patient had no history of carbon monoxide exposure. The Masimo Rainbow SET device recorded an SpCO level of 33%, which was later confirmed with an invasive laboratory measurement. After interviewing the woman, clinicians learned that her utilities had been shut off and she was running a gas-powered generator in her basement.
In the report, researchers said that since early CO toxicity shares symptoms with other more common illnesses, "physicians must maintain a high index of suspicion to avoid incorrect diagnosis, management and disposition. Unrecognized CO poisoned patients returned to the site of exposure may develop more serious CO toxicity." They added that the noninvasive testing provided by Masimo Rainbow SET technology "is a rapid, inexpensive method for screening large numbers of patients for CO toxicity and identifying unsuspected cases that might otherwise be missed."
Finally, a group of researchers
at the Erlanger Health System in Chattanooga, TN used the Masimo Rainbow SET technology to assess CO levels on 136 patients who presented to the outpatient pulmonary lab for arterial blood gas (ABG)
draws to evaluate patient's smoking history as well as 21 patients who presented with burns and inhalation injuries in the ED who also received ABGs. As a result of these tests, the researchers
concluded that the Masimo Rainbow SET Pulse CO-Oximeter "performed well in both the pulmonary and the Emergency Department environments, with an extremely small bias compared to CO-oximetry measured
COHb." They added that based on their study, the technology was "quite reliable at detecting elevated CO levels in patients presenting to the pulmonary lab or emergency department."
About
Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and
Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical
studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the "gold standard" pulse
oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon
monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcome and
Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Evaluation of a New Pulse CO-Oximeter: Noninvasive Measurement of Carboxyhemoglobin in the Outpatient Pulmonary Lab and Emergency Departments. Layne T, Snyder C, Brooks D, Enjeti. Pulmonary Physiology Department, Erlanger Health System, Chattanooga, TN.
Non-Invasive Carboxyhemoglobin Monitoring: Screening Emergency Department Patients for Carbon Monoxide Exposure. Partridge R, Chee KJ, Suner S, Sucov A, Jay G. Department of Emergency Medicine, Rhode Island Hospital, Brown Medical School, Providence, RI.
Unsuspected Carbon Monoxide Toxicity Detected by Non-Invasive Monitoring: A Case Report. Partridge R, Chee KJ, Suner S, Sucov A, Jay GD. Department of Emergency Medicine, Rhode Island Hospital, Brown Medical School, Providence, RI
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow, SpCO, SpMet and Pulse CO-Oximeter are trademarks of Masimo Corp.
Breaking Study: Masimo Blue Sensor Proven Most Accurate in Measuring Oxygen Saturation on Babies with Cyanotic Heart Disease
New study presented at the 2006 Respiratory Care Congress concludes that Masimo Blue sensor is only sensor to demonstrate acceptable accuracy on this low saturation patient population
Irvine, California December 13, 2006 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that a new independent study presented today at the 2006 American Association for Respiratory Care (AARC) Congress in Las Vegas, concluded that the Masimo Blue sensor is the most accurate technology for monitoring babies with cyanotic congenital heart disease. This patient population has represented a difficult challenge for pulse oximeters due to the patients' unique physiology and the need to keep their saturation at a low level. The researchers compared Masimo SET Radical using the Masimo Blue sensor with the Nellcor N600 LoSat technology using a Max-I sensor and concluded that only the Masimo Blue sensor demonstrated acceptable accuracy on this patient population.
The study entitled, "New Pulse Oximetry Sensors with Low Saturation Accuracy Claims - A Clinical Evaluation" was conducted at The Toronto Hospital for Sick Children by a research team headed by Dr. Peter Cox. The researchers indicated that, despite many advances in pulse oximetry technology, accuracy on patients with low saturations was still a problem. Dr. Cox's specific interest was the performance of pulse oximeters on babies with cyanotic congenital cardiac lesions (CCCL) which typically are kept at very low blood oxygen levels to maintain a balance of blood flow to the lungs and the body. He explained that careful maintenance of oxygen saturation levels on these babies is critical to their survival. The study was prompted by the fact that two pulse oximetry manufacturers have recently introduced products specifically suited for this patient population. Masimo introduced the Blue sensor in 2005 and Nellcor introduced their LoSat technology in 2006.
Dr. Cox and his team compared the Masimo Blue sensor to a Nellcor N600 with LoSat technology on babies with CCCL. The researchers found a statistically significant difference between the Masimo and Nellcor pulse oximeters and concluded, "Despite advances in technology, only the new Masimo Blue sensor demonstrates acceptable accuracy as demonstrated by a smaller bias, precision and Arms."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995,
the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening
events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the
technology as the "gold standard" pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time,
noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the
mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow, SpCO, SpMet and Pulse CO-Oximeter are trademarks of Masimo Corp.
New Pulse Oximetry Sensors with Low Saturation Accuracy Claims - A Clinical Evaluation. Cox PN. Hospital for Sick Children, Toronto, Canada, 2006.
Dräger Medical and Masimo Announce Expanded Relationship
Dräger Medical adopts Masimo Rainbow SET as its standard oximetry platform. The two companies also released each other from any potential patent infringement claims on pulse oximetry
Irvine, California-November 8, 2006-Dräger Medical AG & Co. KG today announced that it has expanded its relationship with Masimo and will integrate Masimo Rainbow SET platform as its principal pulse oximetry technology. The Rainbow SET platform offers Masimo SET Read-Through Motion and Low Perfusion pulse oximetry plus upgradeability to add other parameters in the future. Upgrades are available today for carboxyhemoglobin (carbon monoxide) and methemoglobin and others are planned for the future. Dräger Medical will be incorporating Masimo Rainbow SET into major acute care products. For future product developments, Dräger Medical will replace its Oxisure+ oximetry with Masimo technology. This agreement between Dräger Medical and Masimo also constitutes a release of each other from any potential patent infringement claims relating to pulse oximetry.
Dr. Wolfgang Reim, President and CEO of Dräger Medical stated, "We are excited to be able to extend our existing relationship and again be one of the first companies to provide our customers with the latest advancements in noninvasive patient monitoring technology. Masimo SET is clearly the gold standard in motion tolerant pulse oximetry technology and with its new Rainbow Technology Masimo extends the reach of its technology to new important parameters. Dräger Medical and Masimo have enjoyed a great partnership that we are very happy to expand."
Joe E. Kiani, Chairman and CEO of Masimo stated, "We greatly value our relationship with Dräger Medical and are happy to be expanding our relationship with this new agreement. Dräger Medical's leadership in patient monitoring and therapeutic systems is well recognized by numerous customers around the world. We are delighted to see Dräger Medical taking Masimo Rainbow SET to its customers, and thereby improving care of millions of patients."
About Dräger Medical
Dräger Medical AG & Co. KG is one of the world's leading manufacturers of medical equipment, the largest division of Drägerwerk AG (history dates back to 1889) and a 65:35 joint venture company between Drägerwerk AG and Siemens AG. The global Company offers products, services and integrated CareArea™ Solutions throughout the patient care process - Emergency Care, Perioperative Care, Critical Care, Perinatal Care and Home Care. With headquarters in Lübeck, Germany, Dräger Medical employs nearly 6,000 people worldwide, around half of whom work in customer sales & services. R&D and production are located in Lübeck, Germany; Best, Netherlands; Telford, PA, and Andover, MA, USA; and Shanghai, China. The Company has sales and service subsidiaries in almost 50 countries and is represented in more than 190 countries. Dräger Medical provides innovative solutions for acute patient care, which are the result of a clear focus on core competencies, a close dialog with customers, over a century of experience in the market, and continuous investment in R&D. Additional information is available on the Company's website www.draeger.com
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the "gold standard" pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Claudia Büring, Head of Public Relations at Dräger Medical
Phone: +49-451-882-1986
Email: claudia.buering@draeger.com
Tom McCall, Vice President, Corporate
Communications at Masimo Corporation
Phone: 1- 949-297-7075
Email: tmccall@masimo.com
Breaking Studies: Masimo SET Pulse Oximetry Technology Again Shown to be Most Effective
New studies presented at last week's 2006 ASA Annual Meeting add to the more than 100 independent studies validating Masimo SET as the gold standard; technology is the foundation that enables Pulse CO-Oximetry
Irvine, California October 27, 2006 - Masimo, the inventor of Pulse CO-Oximetry and read-through motion and low perfusion pulse oximetry, reported that multiple independent studies were presented last week at the 2006 American Society of Anesthesiology (ASA) Annual Meeting in Chicago, each reinforcing the superiority of Masimo SET in providing accurate, reliable pulse oximetry readings. In the studies, Masimo SET was shown to "work better for patient safety" during the most difficult clinical conditions of motion and low peripheral perfusion.
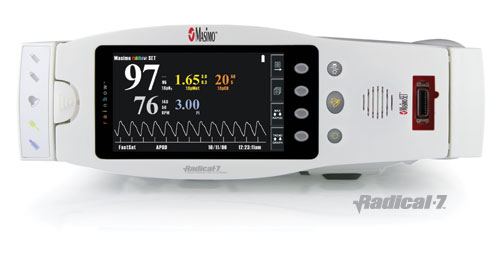
Masimo SET is the foundational technology that allowed the company to introduce Masimo Rainbow SET Pulse CO-Oximetry-the first and only monitoring platform that allows for the continuous and noninvasive measurement of carbon monoxide (COHb), methemoglobin (MetHb), oxygen saturation (O2Hb), pulse rate and perfusion index. Masimo introduced the first bedside monitors, Radical-7, to feature this groundbreaking technology at last week's ASA meeting, and hosted a pre-ASA symposium on clinical implications of noninvasive and continuous monitoring of MetHb and COHb using Masimo Rainbow SET. Specific findings of the studies include:
Masimo shown to be "better for patient safety"
In a study entitled "Comparison of Three New Generation Pulse Oximeters during Motion & Low Perfusion in Volunteers" performed by Nitin Shah, MD and Laverne Estanol, MS at the VA and UC Irvine Medical Centers in Long Beach, CA, the researchers stated that pulse oximeter accuracy is often compromised by low perfusion states and motion artifact that can jeopardize patient safety in the OR, PACU, and ICU, adding that "manufacturers keep improving their technology in an attempt to solve this problem". To asses the effectiveness of the newest technologies, the study compared Masimo SET with the Nellcor N-600 and GE Datex-Ohmeda TruSat, under conditions of low perfusion and motion in hypoxic and normoxic states in volunteers between the ages of 18 and 40 years old.
The results showed that Masimo had the lowest level of false alarms, performing nearly six times better than the Nellcor N600. For the rate of missed true events, the Masimo unit again had the lowest level, performing 17 times better than the Nellcor N600. The researchers concluded that during hypoxic/normoxic and low perfusion states, "Nellcor N-600 and Datex Ohmeda TruSat performed inferior to Masimo Radical with respect to maintaining accurate readings during both machine generated and self generated motions". They added that "it appears from this study that Masimo Radical may work better for patient safety, especially at critical times in OR, PACU, and ICU."1
In a separate report entitled "Impact of Motion and Low Perfusion on SpO2 & Pulse Rate in Three New Generation POs in Volunteers", Shah and Estanol found that the Masimo SET device was within 7 percent of the true saturation measurement 98 percent of the time as compared to 72.7 percent for the Nellcor N-600, concluding that Masimo "performed the best in this vigorous testing schedule for both SpO2 and pulse rate" and added that "Masimo will give reliable SpO2 & PR values for a greater period of time as compared to Datex-Ohmeda TruSat and Nellcor N-600 in the OR, PACU, and ICU". 2
In another abstract, entitled "Failure Rates & Recovery Times of New Generation POs during Motion and Low Perfusion in Volunteers", Shah and Estanol explained that patient movement and low perfusion due to lower temperature is common in the PACU and OR, especially during extubation. They examined the failure rate, the percentage of times that the monitor was more than 7 percent off from the actual SpO2 reading, and the recovery time, the average amount of time taken for the monitor to return to accurate values. The most significant finding was the difference in SpO2 failure rates with Masimo SET was more than 10 times better than Nellcor N-600, concluding that "Masimo may serve better for patient safety."3
Masimo accuracy cited as beneficial to children with cyanotic congenital heart disease
Additional studies presented at the ASA included "The Accuracy of Masimo SET and Nellcor N-595 in Children with Cyanotic Congenital Heart Disease" conducted by Yuichiro Toda, M.D., Mamoru Takeuchi, M.D., Tatsuo Iwasaki, M.D., Kazuyoshi Shimizu, M.D., Kiyoshi Morita, M.D. from Okayama University Medical School in Japan. In this study, the researchers compared the performance of Masimo SET and specifically Masimo's Blue sensor to the Nellcor 595 and the Max-I Sensor on infants with cyanotic congenital heart disease, a patient population with a reputation for causing erroneous pulse oximetry readings. The Masimo Blue sensor was specifically designed for this patient population. The researchers found that overall, the bias (error) of the Nellcor sensor was approximately 18 times that of Masimo's, but on the sickest patients with the lowest blood flow, the Nellcor bias (error) was more than 21 times that of the Masimo Blue Sensor prompting the researchers to conclude "Masimo blue sensor presented smaller bias compared with Nellcor sensor" and that "Nellcor presented wider bias during low perfused state than that at normal perfusion". They concluded that of the two technologies, "Masimo blue sensor provides the accurate measurement of pulse oximetry in patients with cyanotic congenital heart disease". 4
These and all the studies referencing the superiority of Masimo technology were presented in abstract form at the ASA Annual Meeting and are available for viewing on the ASA website at www.asa-abstracts.com.
Joe E. Kiani, Chairman & CEO of Masimo stated; "It is gratifying to see so many independent researchers taking the time to evaluate Masimo SET and to communicate the positive impacts on patient care and safety that can be realized through its use. We are confident that the introduction of Masimo Rainbow SET will further enable clinicians to do what's right for patient care by giving them a more accurate picture of their patients' status with the continuous and noninvasive monitoring of oxyhemoglobin, methemoglobin, carboxyhemoglobin, perfusion index and pulse rate."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations- establishing the technology as the "gold standard" pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
- Nitin Shah, M.D., Laverne Estanol, M.S. Anesthesiology, Long Beach VA Medical Center; UC Irvine Medical Center, Long Beach, California. Anesthesiology 2006; 105: A929
- Nitin Shah, M.D., Laverne Estanol, M.S. Anesthesiology, Long Beach VA Medical Center; UC Irvine Medical Center, Long Beach, California. Anesthesiology 2006; 105: A1433
- Nitin Shah, M.D., Laverne Estanol, M.S. Anesthesiology, Long Beach VA Medical Center, UC Irvine Medical Center, Long Beach, California. Anesthesiology 2006; 105: A242
- Yuichiro Toda, M.D., Mamoru Takeuchi, M.D., Tatsuo Iwasaki, M.D., Kazuyoshi Shimizu, M.D., Kiyoshi Morita, M.D., Dept. of Anesthesiology and Intensive care medicine, Okayama University Medical School, Okayama-shi, Okayama-ken, Japan. Anesthesiology 2006; 105: A1704
Contact:
Tom McCall
Masimo Corporation
949-297-7075

Masimo, SET, Signal Extraction Technology, Rainbow, Radical, APOD, SpCO, and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Radical-7, Rad-57, SpMet and Pulse CO-Oximeter are trademarks of Masimo Corp.
|
When the security guard arrived in the hospital emergency room, he was dizzy and had a headache -- vague symptoms that a nurse could have brushed off easily. But in this case, the nurse, aided by a new device, triggered a series of events that led to the evacuation of 100 persons from a 20-story condo that had lethal levels of carbon monoxide, perhaps saving untold lives. ''I can't tell you definitely that people could have died, but it came close enough that it shook us up big-time,'' said Mary Russell, an ER nurse at Boca Raton Community Hospital. The hospital is in the area where anthrax hit the National Enquirer offices, and its emergency room, as well as the Boca Raton fire department's hazardous materials medical response unit, now are ready for a broad range of threats. The Boca case began at 1:50 p.m. on Sept. 7. ''We had a very astute charge nurse, and when he mentioned he smelled some fumes, she asked if he had been around generators,'' said Russell, a research preparedness specialist at the hospital. ``He said yeah. Construction was going on in the building. Carbon monoxide was already very much on our radar screen, and we had just gotten this new device, a Masimo Rad-57.'' Until the arrival of this device, testing carbon monoxide levels in humans was a long and painful process, involving the removal of blood from an artery and getting a lab result. ''That is exquisitely painful,'' said Russell. ''Trust me you don't want to do it.'' For that reason, most nurses avoid giving the test unless it's absolutely necessary. But Boca had recently purchased the Rad-57, for about $3,000, which measures carbon monoxide levels by simply attaching a sensor to a finger tip. The first device of its kind, it was introduced less than a year ago, says Tom McCall of the California-based Masimo. In the case of the security guard, his levels were extremely high. The hospital called Boca Raton Fire Rescue, which rushed its HazMed unit to the building at 2800 S. Ocean Blvd. ''They got a reading of 900 parts per million in the lobby,'' said Glenn Joseph of Fire Rescue. ''That's 100 times higher than normal.'' Other areas showed readings of 500. The condo had been undergoing hurricane repairs, and the construction crews had generators going in the garage area, said Joseph. ``We had them stop all operations.'' Rescue crews went floor by floor, telling the 100 or so persons in the building they needed to leave. Only one other person was taken to the hospital, said Russell. That person and the security guard were given oxygen and recovered quickly. FLU-LIKE SYMPTOMS For ER nurses, the problem is that carbon monoxide poisoning can often present itself merely as flu-like symptoms or food poisoning. Once in the ER, patients can tend to recover quickly since they're no longer near the fumes -- complicating the ability to discover the cause. McCall of Masimo pointed to news reports in June from Ocean City, Md., where several persons in a Days Inn were taken to an emergency room about 9:30 a.m. Neither the ER nor the rescue units thought to check for carbon monoxide, and their illness was blamed on food poisoning. At 2 p.m., a 40-year-old Pennsylvania tourist and his 10-year-old daughter were discovered dead in an adjacent room. At least one of them was still alive earlier in the day when the first persons complained of feeling ill, according to local news reports. UNITS IN FLORIDA Masimo now has 41 Rad-57 units in the field in Florida, either in emergency rooms or possessed by ''first responders'' to the scene, McCall said. The hospital in Boca Raton has learned to be ready for just about anything. It tested dozens of persons for anthrax after the mail scare in 2001, and after hurricanes Frances and Jeanne, it saw ''whole families transported to us'' with carbon monoxide poisoning caused by generators, said Russell. ''I think there's a lesson to be learned here about generators,'' she added. She suggested construction inspectors should do more to check for the location of generators in buildings, and she would like to see stores like Home Depot have displays for home carbon monoxide sensors (which can be as cheap as $30 to $45) right next to the generators. She said she tried to find out how many generators were now in the state and had no luck. ''I can't even tell you how much this scares me,'' Russell said. Contact: JOHN DORSCHNER Copyright 2006 Miami Herald Media Co. |
|
| ||||||||||||||
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events.
More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Masimo SET with Rainbow Technology and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact: Tom McCall
Masimo Corporation
949-297-7075
tmccall@masimo.com
Masimo Announces FDA Clearance of Radical-7, First Bedside Monitor with the Breakthrough Rainbow Technology
Masimo SET with Rainbow technology is the first and only continuous and noninvasive carboxyhemoglobin, methemoglobin, and oxyhemoglobin saturation monitor
Irvine, California October 5, 2006 --Masimo today announced it has received FDA clearance for Masimo Radical-7--the first bedside monitor to feature the award winning Rainbow technology--which will be introduced at the upcoming Annual Meeting of the American Association of Anesthesiologists October 14-18 in Chicago. Two new Radical-7 bedside monitors with different user interfaces including a color display will be available with Masimo SET with Rainbow technology, the first and only way for clinicians to continuously and noninvasively monitor their patients' carboxyhemoglobin (SpCO), methemoglobin (SpMet), oxygen saturation (SpO2), pulse rate, and perfusion index.
Masimo SET with Rainbow Technology has already proven to be effective in detecting carbon monoxide and methemoglobin poisoning in critical situations, allowing accurate diagnosis and early treatment of life-threatening conditions. Last month at a hospital in Southern California, a patient was diagnosed with a Rainbow monitor to have a dangerously high level of methemoglobin. Because of this timely diagnosis, the patient was immediately treated and the patient's life was saved.
Also, last month a hospital in Boca Raton, Florida used a hand-held Rainbow monitor, the Masimo Rad-57, to avert a potential disaster. Clinicians at the hospital, after using the Rad-57 to detect dangerous levels of carbon monoxide in a patient's blood, dispatched emergency services to the young man's condo complex and safely evacuated nearly 200 people from a 20-story building where a faulty generator led to carbon monoxide levels 100 times greater than normal. As a result, there were no reports of permanent injury to any of the building's residents.
"We are proud of the scientific achievement in making noninvasive and continuous measurement of SpCO and SpMet possible, but life-saving incidents like the ones noted above bring a different kind of satisfaction to those accomplishments," explained Joe Kiani, Masimo Chairman and CEO.
The Rainbow platform is based on Masimo's revolutionary and gold standard Signal Extraction Technology --the world's first and highest performance pulse oximetry technology clinically proven accurate and reliable during periods of patient motion and low peripheral perfusion. But while other pulse oximetry technologies use only two wavelengths of light to distinguish oxygenated from nonoxygenated hemoglobin, Masimo SET with Rainbow technology uses multiple (7+) wavelengths to noninvasively and continuously measure carboxyhemoglobin and methemoglobin in addition to oxygen saturation, pulse rate and perfusion index. In addition, when used with Rainbow sensors, the Rainbow monitors employ a new probe-off technology called RAPOD, which can detect when the sensor has come off the patient more reliably than ever before.
Rainbow allows clinicians to be more confident of the accuracy of their SpO2 readings by giving them the ability to frame those measurements with the dyshemoglobins SpCO and SpMet. Neither SpCO nor SpMet can be distinguished from, and are often reported as, SpO2 by all other pulse oximeters, yet both are incapable of transporting oxygen, resulting in reduced blood oxygenation levels that can induce tissue hypoxemia. Peer-reviewed clinical studies have proven that the prevalence and significance of both these dyshemoglobins raise morbidity and mortality across the spectrum of acute care settings.
The Institute for Safe Medical Practice (ISMP) states that "methemoglobinemia is unlikely to be a rare occurrence", while authors from the 2004 Johns Hopkins University School of Medicine study[i] entitled "Acquired Methemoglobinemia", concluded "drugs that cause acquired methemoglobinemia are ubiquitous in both the hospital and the outpatient setting." The Johns Hopkins study had many key findings:
- Acquired methemoglobinemia is ubiquitous in hospitals, from OR to the General Ward and is independent of patient's age, from 4 days of age to 86 year of age.
- More than 20 drugs that are used frequently in hospitals cause acquired methemoglobinemia, including 'caine' anesthetics such as Benzocaine and Lidocaine, heart medications, such as nitroglycerin, inhaled nitric oxide used on premature infants and sometimes cardiac patients, and Dapsone, a powerful anti-infective which is commonly used on organ transplant, AIDS, and dermatoses patients.
- Methemoglobinemia can cause serious injury and even death, but can be treated if detected. During the study time, there were three near deaths and one death.
- Nearly 20% of patients tested had elevated methemoglobin levels and 25% of the cases were found accidentally.
- The cost of doing invasive testing of methemoglobin is $25 each time and during the 28-month period it would have cost the hospital $9 Million.
"Before the advent of Masimo SET and Rainbow Technology, it was impossible for clinicians to reliably monitor their patients continuously for methemoglobinemia and carboxyhemoglobinemia, let alone oxygen saturation, and as a result many patients suffered. We are proud to once again bridge the gap between measurement and patient condition, by breaking the technological barriers and introducing SpCO and SpMet," Kiani continued. "But in addition to the current ability to monitor the level of SpCO and SpMet, Masimo is using the additional data delivered by the Rainbow technology platform and sensors to qualify an array of additional clinically valuable measurements. And because Masimo SET with Rainbow technology is designed as a technology platform, hospitals can easily upgrade to these additional new noninvasive clinical measurements when they become available without acquiring new hardware."
The Radical-7 is the first pulse oximeter to have a color screen. Additionally, to provide customers with maximum flexibility in their transition to Rainbow technology, each Masimo Radical-7 is fully field upgradeable, so customers can purchase the Radical-7--which comes standard with Masimo SET SpO2, pulse rate and perfusion index--with Rainbow parameters at the time of purchase, or have them field installed in the future. In June, Masimo received a prestigious Medical Design Excellence Gold Award for its Rad-57 handheld Pulse CO-Oximeter with Rainbow technology. In addition, the technology received the 2006 Application of Technology award from the Society for Technology in Anesthesia in January and was honored by the American Electronics Association as the Innovative Product winner in the Medical Technology category at its Thirteenth Annual High Tech Awards ceremony in May.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, RAPOD, and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow, SpCO, SpMet and Pulse CO-Oximeter are trademarks of Masimo Labs.
Contact: Tom McCall
Masimo Corporation
949-297-7075
[i] Ash-Bernal R, Wise R, Wright SM. Acquired Methemoglobinemia. A Retrospective Series of 138 Cases at 2 Teaching Hospitals. Medicine 2004; 83: 265-272.
New Masimo Rainbow SET™ Pulse CO-Oximetry™ Technology Receives Prestigious Medical Design Excellence Gold Award
Irvine, California September 5, 2006 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced it has received a prestigious Medical Design Excellence Gold Award for its innovative new Masimo Rainbow SET Rad-57 Pulse CO-Oximeter.
The Masimo Rad-57 Pulse CO-Oximeter is the first and only device that allows clinicians to detect and continuously monitor carbon monoxide levels in the bloodstream non-invasively. In clinical studies and in the field, Masimo Rainbow SET is already proving itself effective in detecting carbon monoxide poisoning in seconds, allowing accurate diagnosis and early treatment of a life-threatening problem that is frequently misdiagnosed as flu or migraine. Masimo has also recently received FDA clearance for the noninvasive measurement of methemoglobin levels in the blood. A recent Johns Hopkins study found that methemoglobinemia, a potentially lethal condition that starves the tissues of oxygen, is much more common in hospitalized patients than previously realized.
Joe E. Kiani, Chairman & CEO of Masimo stated, "We are honored to receive this Medical Design Excellence Gold Award. We received a similar award years ago when we introduced the Radical Signal Extraction Pulse Oximeter, which was the first technology to measure oxygen saturation and pulse rate during motion and low perfusion. We take much pride that the second breakthrough in oximetry is once again developed by our engineering team. With our new Masimo Rainbow SET platform, we are collecting a much more rich data stream separated from interference, enabling us to distinguish additional blood constituents. Our clinical and engineering teams are currently working to identify and qualify additional Rainbow parameters. We thank the organizers of the MDEA program for this award and their recognition of this innovative platform, and I thank our engineering team for their brilliant efforts and tireless progress."
The Medical Design Excellence Awards competition is organized and presented by Canon Communications LLC (Los Angeles) and is the only awards program that exclusively recognizes contributions and advances in the design of medical products. Entries are evaluated on the basis of their design and engineering features, including innovative use of materials, user-related functions that improve healthcare delivery and change traditional medical attitudes or practices, features that provide enhanced benefits to the patient, and the ability of the product development team to overcome design and engineering challenges so the product meets its clinical objectives. A comprehensive review of the entries was performed by an impartial, multidisciplinary panel of third-party jurors with expertise in biomedical engineering, human factors, industrial design, medicine, and diagnostics.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 70 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact: Tom McCall
Masimo Corporation
949-297-7075
American Society of Anesthesiologists Issues New Practice Guidelines for the Perioperative Management of Patients with Obstructive Sleep Apnea
Masimo announces new Patient Safety Net System that together with Masimo SET's unmatched sensitivity and specificity will help hospitals comply with the ASA guidelines
Irvine, California, August 24, 2006 - The American Society of Anesthesiologists (ASA) recently adopted practice guidelines for the perioperative management of patients with obstructive sleep apnea (OSA) to reduce the risk of adverse outcomes in patients with OSA who receive sedation, analgesia or anesthesia. OSA is a syndrome characterized by periodic, partial, or complete obstruction of the upper airway during sleep. It is well known that even patients without a history of OSA can develop OSA during perioperative phases. OSA patients are especially vulnerable during the postoperative period because protective arousal reflexes are diminished due to the effects of anesthesia and opioid analgesia. The ASA guidelines recommend preoperative screening of all surgical patients for OSA followed by a postoperative protocol of continuous pulse oximetry monitoring until the patient is no longer at risk.
Masimo, the inventor and pioneer of Read-Through Motion and Low Perfusion Pulse Oximetry, has recently introduced the RadNet Patient Safety Net System to help clinicians monitor their patients continuously and remotely. RadNet provides surveillance for up to 28 patients by either hardwired or wireless connectivity from point of care monitors to a base monitoring station. RadNet is an easy to install, easy to use system that is scaleable based on a specific hospital's needs.
The key to the success of the RadNet system is Masimo SET pulse oximetry technology, proven through more than 80 independent and objective published clinical studies to be the most reliable and accurate pulse oximeter during challenging conditions such as patient motion, low perfusion and bright lights, which can confuse other pulse oximetry technologies. Masimo's patented technology has been proven to dramatically reduce false alarms and improve detection of true alarms, especially in the challenging conditions, such as those encountered in monitoring ambulatory patients in the general care areas.
Steve Moreau, CEO of San Antonio Hospital stated, "High quality patient care and cost savings go hand in hand. When clinicians detect problems early, lives can be saved, patients will recover sooner and health care costs are lowered. Rapid Response Teams have been created to react more quickly to adverse events, but their success is dependent upon reliable and continuous monitoring of patients at risk. Technology solutions such as Masimo SET and RadNet play a critically important role in providing an early warning system for the clinicians providing care at the bedside."
Joe E. Kiani, CEO and Chairman of Masimo stated, "We applaud the ASA for recognizing the need to better care for patients post operatively and we are proud that we have created the tools to help make these recommendations realizable. Masimo SET has clearly been proven as the most reliable pulse oximeter in the world, especially in the presence of patient motion and low perfusion. We developed Signal Extraction Technology to allow patients to be monitored reliably in the general floor. In fact, since our founding in 1989, our mission has been Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications. We are delighted that our mission is consistent with the healthcare community's goals of reducing unnecessary morbidity and mortality."
Recent data suggests that the prevalence of OSA may be much greater than previously thought. A recently published study performed at Barnes Jewish Hospital in St. Louis screened 1,898 surgical patients over four months and found that over 19% of those patients had OSA.
These guidelines add to a significant existing challenge that hospitals have been coping with to better care for recovering patients after being transferred out of the intensive care units (ICU) in order to reduce the incidence of unexpected adverse patients events, often referred to as "sentinel events". This task is made difficult due to the fact that, in most cases, the ICUs are the only areas equipped to provide this level of postoperative care. Not only is there a lack of monitoring outside of the ICUs, but there are also far fewer nurses per patient. So, even if the hospital can put pulse oximeters on all of these patients, there may not be enough nurses to react to the alarms. Making this challenge even more difficult is the fact that most of the existing pulse oximeters are prone to excessive false alarms and missed true events. In a recently completed study that will be presented at the ASA's annual conference this October, the newest pulse oximeters from two of the largest manufacturers had false alarm rates of 17-28% and missed events incidence of 42-82%, while Masimo SET's false alarm rate and incidences of missed events was nearly an order of magnitude lower.
Along with RadNet, Masimo is introducing PPO+, a wireless, wearable Masimo SET pulse oximeter with optional ECG, which will allow patients that are ambulating to have their oxygen saturation, pulse rate and ECG continuously monitored via RadNet wirelessly. There are currently a variety of point of care devices that are compatible with RadNet. Masimo is planning to unveil new Masimo SET and Masimo Rainbow SET enabled monitors to further serve the growing needs of care providers, especially those in the general ward.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient
care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased
pulse oximetry's ability to detect life-threatening events. Over 80 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation
in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin
in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care
by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Masimo, SET, Signal Extraction Technology and Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo
Corporation. RadNet, Patient Safety Net System and PPO+ are trademarks of Masimo Corporation. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact: Tom
McCall
949-297-7075
tmccall@masimo.com
Masimo and Respironics Announce an Expanded Relationship, the Adoption of Masimo Rainbow SET Pulse CO-Oximetry, and Settlement of Patent Dispute
Irvine, California - August 1, 2006, Respironics, Inc. today announced that it has decided to expand its relationship with Masimo and to adopt Masimo's pulse oximetry solution for situations requiring read through motion technology across all of its business units. Until now Respironics has only used Masimo SET in certain of its sleep and respiratory products. Respironics will be gradually incorporating Rainbow SET in all Respironics products where the improved capabilities will provide clear clinical improvements. Respironics will be phasing out its Novametrix MARS oximetry product line over time but will continue to service customers until a comprehensive plan is in place. This agreement between Respironics and Masimo also constitutes a release by Masimo of Respironics and its affiliates from certain patent infringement claims and provides Respironics with access to the Masimo Rainbow SET technology.
Craig Reynolds, COO of Respironics stated, "Masimo SET is clearly the gold standard in pulse oximetry technology for its ability to reliably measure oxygen saturation and pulse rate during difficult situations, but with its new Rainbow Technology, Masimo further advances the science by being able to simultaneously measure carboxyhemoglobin and methemoglobin noninvasively, which until now could only be measured invasively. Masimo and Respironics have enjoyed a great business relationship for over 10 years. We are excited to be able to extend our existing relationship and again be one of the first companies to provide our customers with the latest advancements in noninvasive patient monitoring technology."
Joe E. Kiani, Chairman and CEO of Masimo stated, "We greatly value our relationship with Respironics and are happy to be expanding our relationship with this new agreement. Respironics was one of the first OEM users of Masimo technology, taking our revolutionary read-through motion and low perfusion pulse oximetry technology to the sleep labs and home care when that was the business they focused on. We are delighted to see Respironics not only make great headway in the hospital market with its ventilator products, but to take Rainbow SET to its customers around the world."
Respironics stated that it will not be changing its financial outlook for guidance based on the events described in this press release.
About Respironics, Inc.
Respironics is a leading developer, manufacturer and distributor of innovative products and programs
that serve the global sleep and respiratory markets. Focusing on emerging market needs, the Company is committed to providing valued solutions to help improve outcomes for patients, clinicians and health
care providers. Respironics markets its products in 131 countries and employs over 4,600 associates worldwide. Further information can be found on the Company's Web site: www.respironics.com
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted
Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over
70 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced
Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially
life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
Contact:
Maryellen Bizzack
724-387-5006
maryellen.bizzack@respironics.com
Masimo Renews Agreement with Amerinet for Masimo SET Pulse Oximetry and Adds New Masimo Rainbow SET™ Technology
IRVINE, California - July 27, 2006 - Masimo Corporation today announced that it has renewed a three-year, dual source agreement with Amerinet, a leading health care group purchasing organization with more than 25,000 members ranging from large health systems to physician's offices to long-term care facilities. The agreement continues coverage of Masimo SET® pulse oximetry technology and adds the company's breakthrough Masimo Rainbow SET Pulse CO-Oximetry™ in standalone monitoring devices, handhelds and sensors.
"We are pleased to announce the renewal of our agreement with Amerinet," explained Masimo Chairman and CEO Joe E. Kiani. "And we believe that Amerinet members will be pleased to continue to have access to superior Masimo SET pulse oximetry products as well as the new Masimo Rainbow SET technology that will allow for the earlier detection and treatment of an expanding number of potentially life-threatening conditions."
Masimo SET technology allows clinicians to obtain accurate pulse oximetry readings even in situations of motion and low-perfusion, and has been proven more accurate and reliable in the most challenging clinical settings by over 70 peer reviewed published clinical studies. Building on this technology platform, Masimo has recently introduced Masimo Rainbow SET, world's first device capable of noninvasively measuring carboxyhemoglobin, methemoglobin and oxyhemoglobin saturation levels in the blood
In clinical studies and in the field, Masimo Rainbow SET is already proving itself effective in detecting carbon monoxide poisoning in seconds, allowing accurate diagnosis and early treatment of a life-threatening problem that is frequently misdiagnosed as flu or migraine. In addition, a recent Johns Hopkins study found that methemoglobinemia, a potentially lethal condition that starves the tissues of oxygen, is much more common in hospitalized patients than previously realized.
About Amerinet
Amerinet is one of the most innovative and effective
health care group purchasing organizations in the United States, partnering with members to improve their operating margins. More than 2,100 hospitals and 35,000 non-acute care facilities create new
revenue and reduce expenses with Amerinet's tools for group purchasing, supply chain management, clinical consulting, revenue cycle management, and information and education.
About
Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and
Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 100 clinical studies have confirmed
that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for
the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact: Tom McCall
Vice President, Corporate Communications
949-790-7075
tmccall@masimo.com
New Study Concludes That Less Than Half of Hospitals Have Equipment On Site to Diagnose Carbon Monoxide Poisoning
Researchers suggest that a new, lower cost, noninvasive device that accurately measures carbon monoxide in the blood may be the solution
Irvine, California, July 11, 2006 - A recent study published in The Journal of Emergency Medicine titled "Carboxyhemoglobin Measurement by Hospitals: Implications for the Diagnosis of Carbon Monoxide Poisoning", examined the capability for diagnosing carbon monoxide poisoning at 204 acute care hospitals in the Pacific Northwest, concluding that only 44% of the hospitals had the necessary equipment on site to measure carbon monoxide levels in the blood. For the hospitals that did not have the testing equipment, the average time to receive results of a blood sample sent to another facility was over 15 hours.
The study states that carbon monoxide poisoning is common in the U.S. with over 40,000 emergency room visits annually for diagnosed cases. The authors also suggested that it is likely that there are many more undetected cases due to the fact that the symptoms for carbon monoxide poisoning are not obvious and often attributed to other causes. The authors stressed the importance of rapid diagnosis to speed treatment in order to prevent permanent brain injury.
Until recently, the only way to accurately measure the level of carbon monoxide in blood was through a test called CO-oximetry, which requires a blood sample to be taken and run through a laboratory CO-oximeter. The authors in this study found that, if the hospital had a CO-oximeter on site, it took, on average, 10 minutes to perform the test, but if the hospital had to send the sample offsite, it took over 15 hours, on average.
The lead researcher and author of the study, Neil B. Hampson, MD, stated, "There is a new noninvasive pulse CO-oximeter now available from Masimo Corporation that might help this situation. With the relatively high price of an invasive CO-oximeter, I would presume that the lack of availability is usually due to cost of the instrument. Given that the Masimo device is priced significantly lower, more hospitals should be able to afford them. In addition, if the test can be done in seconds, without taking a blood sample, I would expect many more cases of carbon monoxide poisoning to be detected. This could reduce morbidity due to carbon monoxide poisoning."
About
Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion
and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 70 independent clinical
studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it,
Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening
conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
Masimo Recognized as Top 10 Innovator in Medical Device Industry
Masimo's patents are used as the basis for innovation 1,322% more than the industry average
Irvine, California June 28, 2006 - Masimo Corporation, the inventor of Pulse CO-Oximetry™ and Read-Through Motion and Low Perfusion pulse oximetry ranked 7th overall in the Medical Device Industry in ipIQ's 2006 Patent Scorecard™. The Patent Scorecard is an industry-by-industry ranking of corporate innovation and combines a series of indicators to arrive at patent quality, technological strength and breadth of impact. It has historically been published in MIT's Technology Review and tracks the patent portfolios of more than 2,500 of the world's top technology firms.
ipIQ's Current-Impact Index™, or CII, showcases the broader significance of a company's patents by examining how impactful its patents are across the industry and in specific product categories in the most recent product cycles by measuring how frequently a company's patents are referenced as prior inventions by other patents. A CII of 100 represents average frequency. Masimo's CII was calculated to be 1,422, which means that Masimo's patents are referenced 1,322% more often than average. Masimo's CII score was more than 10 times the average for the medical device sector.
"Masimo's performance is stellar both within the medical device space and compared across every sector," stated Eric Gillespie, Executive Vice President and COO of ipIQ. "The Current-Impact Index is a quality measure designed to illustrate the strength of a company's technology portfolio. Masimo's Current-Impact Index was the highest score of the 2,500 plus companies included in the Patent Scorecard."
Joe E. Kiani, CEO and Chairman of Masimo stated, "Masimo was founded on the basis that to improve patient care and reduce cost of care, breakthroughs were necessary in the area of noninvasive vital signs monitoring. All of our peers were focusing on engineering longer battery life and smaller foot print conventional monitors, and while that is a worthy effort, we believed it should not be put in front of the real task of making accurate and reliable noninvasive vital signs monitors, which required inventions. We set out to solve a problem that the industry thought was unsolvable and our innovations have changed clinicians' expectations of noninvasive monitors and dramatically improved patient care and reduced cost of care."
Mike Petterson, Vice President of Clinical Research of Masimo stated, "Our first product line, Masimo Signal Extraction Technology (SET)® pulse oximetry was the first pulse oximetry FDA cleared for accuracy during patient motion and low perfusion and has ushered in a new performance standard for this critical vital sign. Last year, we introduced the second revolution in noninvasive vital signs monitoring, which we call Rainbow™. Like pulse oximetry, Rainbow is a spectroscopy based platform, but instead of using only two wavelengths of light, our Rainbow platform utilizes multiple wavelengths simultaneously. So far, we have two Rainbow measurements, the noninvasive and continuous measurements of carboxyhemoglobin and methemoglobin. We are working on additional noninvasive, continuous measurements and hope to announce others over the next few years."
About Masimo
Masimo develops innovative monitoring technologies that significantly
improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false
alarms and increased pulse oximetry's ability to detect life-threatening events. Over 70 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor
blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon
monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
About ipIQ
ipIQ - Intellectual Property. Intelligence Quotient. - is the world's leading
Intellectual Property advisor to investment banks, technology-driven companies, and governments. With over three decades of experience, ipIQ utilizes proprietary data, tools, analytics, and technology
to leverage patent-based Intellectual Property as an asset class. The Patent Scorecard is published annually.
For additional information on the Patent Scorecared™ and ipIQ, go to www.ipIQ.com. ipIQ™, Science Linkage™, Current-Impact Index™, and Patent Scorecard™ are trademarks of ipIQ - Intellectual Property, Intelligence Quotient and are used with permission.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
Contact:
Jude Reter
312-205-7037
jreter@ipIQ.com
AEA Honors Masimo with Innovative Medical Technology Award
Irvine, California May 23, 2006 - Masimo Corporation, the inventor of Pulse CO-Oximetry™ and Read-Through Motion and Low Perfusion Pulse Oximetry, was honored by the American Electronics Association's (AEA) Orange County Council at its Thirteenth Annual High Tech Awards ceremony. The AEA recognized the Masimo Rainbow SET Rad-57™ Pulse CO-Oximeter™, as the Innovative Product winner in the Medical Technology category.
Masimo is the inventor of read-through motion and low-perfusion pulse oximetry, a technology called Masimo SET®, which has been proven more accurate and reliable in the most challenging clinical settings by over 70 peer reviewed published clinical studies. Building on this technology platform, Masimo has recently introduced Masimo Rainbow SET™, a new technology that uses eight wavelengths of light to allow clinicians to capture and monitor an unprecedented array of patient physiological data, such as oxygen, carbon monoxide and methemoglobin, noninvasively. Masimo Rainbow SET capabilities will be available in Masimo monitors and in multi-parameter patient monitors produced by leading manufacturers.
The Rad-57 Pulse CO-Oximeter, the first FDA cleared Rainbow SET product from Masimo, is the first device that allows clinicians to detect and monitor carbon monoxide levels in the bloodstream non-invasively. In clinical studies and in the field, Masimo Rainbow SET is already proving itself effective in detecting carbon monoxide poisoning in seconds, allowing accurate diagnosis and early treatment of a life-threatening problem that is frequently misdiagnosed as flu or migraine. Masimo has also recently received FDA clearance for the noninvasive measurement of methemoglobin levels in the blood. A recent Johns Hopkins study found that methemoglobinemia, a potentially lethal condition that starves the tissues of oxygen, is much more common in hospitalized patients than previously realized.
Joe E. Kiani, Chief Executive Officer of Masimo stated "We are honored that the AEA has recognized Masimo with this award. From the very beginning, we always believed that our breakthrough signal extraction technologies would be widely applicable. Our first application, Masimo SET Signal Extraction Pulse Oximetry, has rapidly created a new performance standard for this critical vital sign. With our Masimo Rainbow SET platform, we are collecting a much more rich data stream separated from interference, enabling us to distinguish additional blood constituents. Our clinical and engineering teams are currently working to identify and qualify additional Rainbow parameters. We thank the AEA for this award and it's recognition of this innovative platform, and I thank my team for their brilliant efforts and tireless progress."
About Masimo
Masimo develops innovative
monitoring
technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known
as SET, and
with
it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 70 independent clinical studies have confirmed that Masimo
SET technology
allows
clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the
first time, noninvasively
monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
About AeA
AeA, founded in 1943, is a nationwide
trade association that represents all segments of the technology industry
and is dedicated solely to helping our members' top line and bottom line. We do this in partnership with our small, medium,
and large member companies by lobbying governments at the state, federal,
and
international levels, providing access to capital and business opportunities, and offering select business services and
networking programs. For more information, please visit www.aeanet.org.
Masimo and Dolphin Announce Settlement of Patent Dispute
Hawthorne, California - April 27, 2006, Dolphin Medical, Inc. today announced that it will discontinue its Dolphin ONE product line. The discontinuation is part of an agreement between Dolphin and Masimo Corporation, in which Masimo has agreed to release Dolphin and its affiliates from certain patent infringement claims.
About Dolphin Medical, Inc.
Dolphin Medical, Inc. is an OEM manufacturer and distributor of pulse oximetry sensors
and digital pulse oximetry instruments. In addition to being an OEM provider the company in 2003 entered into a North American distribution agreement with ConMed Corporation to distribute a complete
line of Nellcor compatible sensors. The company is headquartered in Hawthorne, CA and also has manufacturing facilities in Jahor Bahru, Malaysia and Hawthorne, CA. For further information on the
company and its complete range of products, please visit, www.dolphinmedical.com.
About Masimo
Masimo develops innovative
monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as
SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 70 independent clinical studies have confirmed that Masimo SET
technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first
time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in
1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may
be found at www.masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring
to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact: Brad Langdale
949-297-7009
blangdale@masimo.com
Fukuda Denshi and Masimo Announce Pulse Oximetry Purchasing and Licensing Agreement
Fukuda Denshi's Dynascope DS-7300 Bedside Monitor is released with Masimo SET®
April 18, 2006 -Fukuda Denshi Co. (Tokyo, Japan) and Masimo (Irvine, CA) today announced a purchasing and licensing agreement under which Fukuda Denshi will integrate Masimo Signal Extraction pulse oximetry technology (SET) into its future patient monitors. Fukuda Denshi is a leader in the research, development, manufacture and sale of patient monitors and medical devices. Masimo is the inventor of Pulse CO-Oximetry™ and Read-Through Motion and Low Perfusion Pulse Oximetry.
Fukuda Denshi and Masimo also announced the availability of the Masimo SET technology in Fukuda Denshi's Dynascope DS-7300 bedside monitor. The DS-7300 will incorporate all monitoring parameters contained in the Masimo SET Radical® pulse oximeter, including Perfusion Index (PI) a relative assessment of blood flow at the monitoring site and Signal IQ™ (SIQ) technology, which allows clinicians to continuously monitor pulse identification and signal quality during low perfusion and patient motion conditions.
Masimo SET technology has been clinically proven in many independent studies to be accurate during patient motion and low perfusion. Studies show that Masimo SET has virtually eliminated false alarms while enhancing the ability to detect true alarms.
Kotaro Fukuda, President of Fukuda Denshi stated, "After a thorough review of all pulse oximetry technologies available in the marketplace, we concluded that Masimo SET is simply the best oximetry technology available for critical care. Masimo SET improves the performance and reliability of pulse oximetry measurement by substantially reducing the problems of motion artifact, low peripheral perfusion and most low signal-to-noise situations. We are proud to be able to offer this breakthrough technology to our customers."
"Fukuda Denshi is a leader in patient monitoring because they consistently seek to provide best-in-class technology
in their monitoring solutions, "said Joe E. Kiani, CEO and Chairman of Masimo. "The Dynascope DS-7300 with Masimo SET is yet another example of Fukuda Denshi's commitment to improving patient outcomes
by giving clinicians access to leading-edge technologies. Fukuda Denshi has a history of leadership in patient monitoring technologies that extends for over half a century. We are honored to have
been selected as Fukuda Denshi's pulse oximetry platform, and to share in their tradition of excellence."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 70 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
About Fukuda Denshi
Fukuda
Denshi Co., Ltd. was founded in 1939 as Fukuda Special Medical Electric Co. by the late Takashi Fukuda (former chairman of the board of directors) who took part in the development of Japan's first electrocardiograph
in 1935. Now, the company has the sales points not only in each region of Japan but also throughout the world, and is well known as "Electrocardiograph's Fukuda" in the world. In addition,
through technical and marketing tie-ups with domestic and foreign enterprises, the company is developing the business from the examination field to therapeutic equipment, home medicine and emergency
lifesaving fields. Fukuda Denshi acquired the approval to the international standard ISO 9001 for quality system in 1995 and the approval to the international standard ISO 14001 for environment
management system in 2003.
Federal District Court Upholds Antitrust Liability Verdict Against Tyco
Tyco found to unlawfully maintain monopoly power and to have utilized unlawful restraints of trade and exclusionary dealing arrangements in the pulse oximetry market
Irvine, California, March 29, 2006 - Masimo, the inventor of Pulse CO-Oximetry™ and read-through motion and low perfusion pulse oximetry, announced that a federal court in Los Angeles has upheld the jury liability verdict that Tyco Healthcare violated the antitrust laws through anticompetitive business practices specifically related to the sale of its Nellcor pulse oximetry products. On March 21, 2005, after a four-week trial, the jury found that Tyco had unlawfully maintained monopoly power, and that Tyco's sole-source agreements, bundling of unrelated products, market-share based compliance pricing contracts and co-marketing agreements with patient monitoring companies were unlawful restraints of trade and exclusionary dealing arrangements. The jury awarded Masimo $140 million in damages.
On March 22, 2006, the Court upheld the liability verdicts, but reversed the jury's findings based on 2 of the 4 specific practices, bundling and co-marketing agreements, and, as a result, set aside the jury's damages award and ordered a new trial on damages. The jury's original findings that Tyco violated Section 3 of the Clayton Act and Sections 1 and 2 of the Sherman Act were upheld by the Court.
Joe E. Kiani, Chairman and CEO of Masimo stated that, "We are happy that the court has upheld the jury's findings that Tyco's market share based pricing and single source contracts are illegal, which we hope will improve access to cost-effective innovative products that improve patient care. In hope of opening the markets even further to healthy competition, we will continue to press the courts on the practices of bundling of unrelated products. Medical products, from drugs, pacemakers to pulse oximeters, should be judged on their own merits and not based on artificial restraints on purchasing placed on the hospitals by large manufacturers. The American people should get the best care possible."
For many years, some large sellers of medical products have used bundling to exclude competition. Tyco, for example, reduces the discount on many other unrelated products if the hospital chooses to purchase just a small number of Masimo's pulse oximeters, even though the hospital continues to purchase just as much or even greater volume of the unrelated products. Thus, the discount on the whole bundle of products has the effect of being completely attributable to the pulse oximeters. The discount lost on the other unrelated products is sometimes large enough that it can even exceed the whole cost of the pulse oximeters. This makes the decision to purchase the product of clinical choice from Masimo very difficult for hospitals. These schemes can not only cause short term risk to certain patients and clinicians, because the particular hospital may have chosen the substandard product, but long term, such bundling practices will drive competition and innovation out of the healthcare space.
About Masimo
Masimo develops innovative monitoring technologies that
significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated
false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 70 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor
blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide
and methemoglobin in the blood, as well as oxygen saturation, pulse rate and perfusion index, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.Masimo.com
Contact:
Brad Langdale
Masimo Corporation
blangdale@masimo.com
(949) 297-7009
Masimo Announces the FDA Clearance of Masimo Rainbow SET Rad-57cm
World's First Device Capable of Noninvasively Measuring Carboxyhemoglobin, Methemoglobin and Oxyhemoglobin saturation levels in the blood
Irvine, California - March 27, 2006 - Masimo, the inventor of Pulse CO-Oximetry™ and Read-Through Motion & Low Perfusion Pulse Oximetry, announced today the FDA clearance of the Masimo Rainbow SET Rad-57cm Pulse CO-Oximeter™. The Masimo Rad-57cm is a handheld, continuous monitor that analyzes data from a sophisticated yet simple-to-apply, 8-wavelength finger sensor to accurately measure arterial oxygen saturation, carbon monoxide, methemoglobin and pulse rate. Building on the success of the Rad-57 launched in 2005, the Rad-57cm adds the ability to measure methemoglobin, another silent killer like carbon monoxide.
Methemoglobinemia compromises the blood's ability to carry oxygen and can be life-threatening in many areas of the hospital, as well as in many settings outside of the hospital. The Institute for Safe Medical Practice (ISMP) states that "methemoglobinemia is unlikely to be a rare occurrence" while authors from The 2004 Johns Hopkins University School of Medicine studyi entitled "Acquired Methemglobenemia", concluded "drugs that cause acquired methemoglobinemia are ubiquitous in both the hospital and the outpatient setting."
The Johns Hopkins study had several key findings:- Acquired methemoglobenemia is ubiquitous in hospitals, from OR to the General Ward and is independent of patient's age, from 4 days of age to 86 year of age.
- Over 25 drugs that are used frequently in hospitals cause acquired methemoglobenemia, including 'caine' anesthetics such as Benzocaine and Lidocaine, nitroglycerin which is commonly used on cardiac patients, EMLA cream for infants and neonates, inhaled nitric oxide used on premature infants and sometimes cardiac patients, and Dapsone, a powerful anti-infective which is commonly used on organ transplant, AIDS, and dermatoses patients.
- Methemoglobinemia can cause serious injury and even death, but can be treated if detected. During the study time, there were 3 near deaths and one death.
- 20% of patients tested had elevated methemoglobin levels and 25% of the cases were found accidentally.
- The cost of doing invasive testing of methemoglobin is $35 each time and during the 28-month period it would have cost the hospital $9 Million.
- Despite the cost, the authors recommended the measurement of methemoglobin every time blood was drawn for arterial blood gas testing and serial testing during treatment.
Methylene blue, a common dye used in imaging procedures, is the standard treatment for methemoglobinemia. Blood transfusion can also be used to save a patient that has very high levels of methemoglobin.
In another studyii by Osaka City University Medical School Researchers entitled "Elevated Methemoglobin in Patients with Sepsis", the authors showed that methemoglobin rises in patients before they go into septic shock. Detecting the onset of septic shock has been one of the most sought after findings in modern medicine. If patients developing sepsis can be diagnosed early enough, they can be treated more effectively, enhancing their chances of surviving this potentially fatal condition.
Meanwhile, in the outpatient setting, many common agents can cause methemoglobinemia such as: inhalation of industrial fumes from automobile exhaust or the burning of plastics and wood; herbicides and pesticides; industrial chemicals such as petrol octane booster; nitrobenzene; nitroethane which is found in nail polish; resins and rubber adhesives; and drugs of abuse.
Joe E. Kiani, Chairman and CEO of Masimo, stated, "We are proud to once again develop a life saving product that was never available before. Monitoring methemoglobin noninvasively should help save hundreds of thousands of lives around the world. The Johns Hopkins study showed that 20% of the patients tested had elevated methemoglobin. These patient ranged from 4 days old to 86 years old and were found all over the hospital, including the OR, NICU, ICU, and General Ward. During their study, they also reported 3 near deaths and one death. More alarming is that 25% of the cases were found accidentally. We believe that noninvasive monitoring of methemoglobin is essential to reducing the number of injuries and deaths."
"Methemoglobinemia is much more common than the healthcare community realizes" said Maribeth Sayre, MD, Director of Medical Affairs of Masimo. "Historically, methemoglobinemia has often been missed because clinical suspicion was necessary and then a blood test using an expensive laboratory co-oximeter was required for confirmation. Only about 50% of US hospitals have a laboratory co-oximeter, so diagnosing elevated and potentially dangerous levels of methemoglobin has been a practical challenge. We believe that Rad 57cm with Masimo Rainbow SET represents a major advance in patient monitoring."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems.
In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening
events. Over 70 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005
Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, as well as oxygen saturation,
pulse rate and perfusion index, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and
Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.Masimo.com
Masimo, Signal Extraction Technology, SET and LNOP are registered trademarks of Masimo Corporation. Signal Extraction and Rad-57 are trademarks of Masimo Corporation. Rainbow, Pulse CO-Oximetry,
SpMet and SpCO are trademarks of Masimo Laboratories.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
i Ash-Bernal R, Wise R, Wright SM. Acquired Methemoglobinemia. A Retrospective Series of 138 Cases at 2 Teaching Hospitals. Medicine 2004; 83: 265-272.
ii Ohashi K, Yukioka H, Hayashi M, Asada A. Elevated Methemoglobin in Patients with Sepsis. Acta Anaesthesiol. Scand. 1998; 42:713-716.
Masimo Supports National Poison Prevention Week
Irvine, CA - March 22, 2006 Masimo announced today that it is poised to support National Poison Prevention Week, which was proclaimed by the President to be March 19 through March 25, 2006.
National Poison Prevention Week helps raise awareness about the dangers of poison exposure. Since 1962 the President traditionally proclaims the third week of March to be National Poison Prevention Week.
The most common cause of death due to accidental poisoning results from exposure to carbon monoxide, an odorless, colorless gas that is produced by products such as grills, gas stoves and generators, wood burning stoves, water heaters, and automobiles. Every year, 3,500 to 4,000 Americans die from carbon monoxide poisoning, and some 40,000 more seek treatment. It is estimated that countless others are unknowingly exposed to dangerous levels of carbon monoxide and even a single exposure to high levels can lead to cardiac and cognitive damage.
Masimo is the inventor of read-through motion and low-perfusion pulse oximetry, a technology called Masimo SET®, which has been proven more accurate and reliable in the most challenging clinical settings by over 70 independent clinical studies. Building on this technology platform, Masimo recently introduced Masimo Rainbow SET™, a new technology that noninvasively measures carbon monoxide and methemoglobin in the blood. Masimo Rainbow SET uses eight wavelengths of light to allow clinicians to detect and monitor these potentially poisonous compounds without drawing blood or puncturing the skin.
The Rad-57 Pulse CO-Oximeter™ family of handheld devices are the first FDA cleared Rainbow SET products from Masimo. In clinical studies and in the field, Rad-57 is already proving itself effective in detecting carbon monoxide poisoning in seconds, allowing accurate diagnosis and early treatment of a life-threatening problem that is frequently misdiagnosed as flu or migraine.
Masimo offers Rainbow SET products to health care facilities worldwide, and recently began working with fire department and emergency medical service (EMS) agencies to bring this new life-saving technology to first responders.
Joe E. Kiani, Chairman and CEO of Masimo, stated "We are proud to make this new technology available to not only hospitals and other traditional health care facilities, but to firefighters, paramedics and other first responders as well. The Rad-57 Pulse CO-Oximeter is handheld and battery operated, so caregivers can take it right to the scene of a problem. We believe that early recognition of carbon monoxide poisoning is essential to reducing the number of injuries and deaths from this all too common form of poisoning."
Masimo's Fire/EMS sales organization will spend Poison Prevention Week demonstrating the Rad-57 at the EMS Today Exposition in Baltimore, MD. The trade show is a vehicle to deliver training and introduce new technology to the nation's emergency medical personnel.
Information about poison exposure and how homes can be made safer is available at the Centers for Disease Control and Prevention website, www.cdc.gov/health/poisoning.html, and the Poison Prevention Week Council website, www.poisonprevention.org. In case of emergency, families should call 911, or contact their nearest Poison Control Center, 24 hours a day, 7 days a week, by calling 1-800-222-1222.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through-Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 100 clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in challenging critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.Masimo.com
Masimo, SET, Signal Extraction Technology, Rad-57 and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
Masimo Signs Agreement with Premier for Masimo SET Pulse Oximetry, and New Masimo Rainbow SET™ Pulse CO-Oximetry
IRVINE, California- March 9, 2006 Masimo today announced that it has signed a three-year, multisource agreement with Premier Inc., a healthcare alliance entirely owned by more than 200 of the nation's leading hospital and healthcare systems. The agreement covers Masimo SET® pulse oximetry and Masimo Rainbow SET Pulse CO-Oximetry™, including standalone monitoring devices, handhelds and sensors.
Masimo is the inventor of read-through motion and low-perfusion pulse oximetry, a technology called Masimo SET, which has been proven more accurate and reliable in the most challenging clinical settings by over 100 peer reviewed published clinical studies. Building on this technology platform, Masimo has recently introduced Masimo Rainbow SET, a new technology that uses eight wavelengths of light to allow clinicians to capture and monitor an unprecedented array of patient physiological data noninvasively. Rainbow SET capabilities will be available in Masimo monitors and in multi-parameter patient monitors produced by leading manufacturers.
The Rad-57™ Pulse CO-Oximeter™, the first FDA cleared Rainbow SET product from Masimo, is a handheld device that allows clinicians to detect and monitor carbon monoxide levels in the bloodstream non-invasively. In clinical studies and in the field, Masimo Rainbow SET is already proving itself effective in detecting carbon monoxide poisoning in seconds, allowing accurate diagnosis and early treatment of a life-threatening problem that is frequently misdiagnosed as flu or migraine. Masimo has announced that it has other new Rainbow SET monitoring capabilities in advanced development and that the ability to noninvasively detect and monitor methemoglobin levels in the blood is pending FDA clearance. A recent Johns Hopkins study found that methemoglobinemia, a potentially lethal condition that starves the tissues of oxygen, is much more common in hospitalized patients than previously realized.
Joe E. Kiani, Masimo's
Chairman and CEO stated, "We are happy to announce this new agreement with Premier. Since being added to the Premier contract in October 2002, our annualized sales to Premier hospitals have increased
by over 7,500%, signifying the demand for our technology. Through this new agreement, Premier hospital members will continue to have access to superior Masimo SET pulse oximetry products as well
as Masimo Rainbow SET technology, that will allow earlier detection and treatment of an expanding number of potentially life-threatening conditions."
About Premier, Inc.
Premier
Inc., helps hospitals accelerate performance on both clinical outcomes and supply chain costs. Premier is a healthcare alliance entirely owned by more than 200 of the nation's leading not-for-profit
hospital and healthcare systems. These organizations operate or are affiliated with nearly 1,500 hospitals and more than 37,000 other healthcare sites. Premier
Purchasing Partners provides an array of services supporting health services delivery including group purchasing totaling more than $25 billion annually in supplies and equipment purchasing, as well
as supply chain and clinical performance improvement services. Premier Healthcare Informatics offers performance measurement, benchmarking,
and reporting products and advisory services supporting quality improvement. Premier Insurance Management Services helps hospitals manage
insurance costs and improve risk management and claims capabilities. Headquartered in San Diego, CA, Premier has offices in Charlotte, NC, Chicago, and Washington, DC. For more information, visit www.premierinc.com.
About Masimo
Masimo
develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse
oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 100 clinical studies have confirmed that Masimo
SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the
first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.Masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
Masimo Introduces the LNOP® Newborn™ Sensor
Irvine, California - March 6, 2006. Masimo, the inventor and leader of Pulse CO-Oximetry™ and read-though motion and low perfusion pulse oximetry announced the debut of the LNOP Newborn pulse oximetry sensor at last week's California Association of Neonatologists Conference in San Diego. The Masimo Newborn sensor is specifically designed with newborn resuscitation in mind. It provides fastest SpO2 and pulse rate readings at maximum sensitivity when connected to any Masimo SET product, Version 4.1.0.1 or higher. It is also designed for the newborn skin. The Masimo Newborn sensor, combined with Masimo SET® or Masimo Rainbow SET™ technology, gives clinicians the high levels of reliability, sensitivity and specificity needed to provide accurate SpO2 and pulse rate readings after birth or during newborn resuscitation.
The American Academy of Pediatrics' 2005 Neonatal Resuscitation Protocol for pre-term infants recommends using a blend of air and oxygen for resuscitation and a pulse oximeter for monitoring oxygenation status. However, it can be challenging to obtain accurate SpO2 measurements because the newborn is often damp, cold, poorly perfused and moving. When the Masimo Newborn sensor is connected to Masimo-equipped technology, it automatically enables the fastest response and highest sensitivity.
As Dr. Neil N. Finer states in the article "Neonatal Resuscitation: Beyond the Basics" (NeoReviews Vol. 6 No.4 April 2005), "During resuscitation, it is beneficial to have minimal averaging of the SpO2 values coupled with maximal sensitivity, and one manufacturer has developed a probe that, when connected, can reprogram the oximeter to function in this manner (Masimo Corp, Hi-Fi Sensor, Irvine, Calif)." In addition to providing optimal performance, the neonatal sensor allows quick application. With a unique combination of a VelAid™ SofTouch® hook-and-loop attachment and adhesive emitter and detector strip, the sensor stays secure even when the site is wet.
Mitchell Goldstein, MD, Dept. of Neonatology, Pomona Valley Hospital Medical Center in Pomona, California, stated, "Time is crucial when infants are struggling for life. Masimo's Newborn sensor helps clinicians maximize the time we can spend caring for patients. During newborn resuscitation, frequently other oximeters don't work. With Masimo, we have been able to successfully track newborn resuscitation."
Maribeth Sayre, MD, Director of Medical Affairs of Masimo, said, "At the request of caring clinicians, we developed this sensor for the specific and challenging conditions found during newborn resuscitation. The Masimo Newborn Sensor allows clinicians to focus their attention on their patients instead of using valuable seconds configuring the monitor to deliver the performance they need in these extreme situations. Masimo continues to partner with leading clinicians to focus its development efforts on providing solutions for the most challenging conditions where pulse oximetry can make a clinical difference."
Masimo develops innovative monitoring technologies that significantly improve patient care - helping solve "unsolvable" problems. In 1995, the company debuted Read-Through-Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 100 clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.Masimo.com
Masimo, SET, Signal Extraction Technology, LNOP and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
New Study Shows that Masimo SET Pulse Oximetry May Be a Valuable Clinical Tool for Diagnosis of Congenital Heart Disease in Newborns
New low cost method for detecting heart defects in newborns could save many lives
Irvine, California, February 22, 2006 - Masimo, the inventor and leader of Pulse CO-Oximetry and read-though motion and low perfusion pulse oximetry, announced that a recent study, published in Acta Peadiatrica, shows that Masimo SET pulse oximetry is effective in detecting duct dependent congenital heart disease in infants. Recently even the Wall Street Journal reported on the gravity of this situation. Many newborns that are sent home die within days or are forever handicapped from congenital cardiovascular defects that are undetected before discharge. At home, these babies seem to be suffering from flu, but they are suffering from congenital cardiovascular defects. If properly detected before discharge, most of these babies can get treatment and lead normal lives.
The study, "Screening for Duct Dependent congenital heart disease with Pulse Oximetry: A critical evaluation of strategies to maximize sensitivity", outlines a relatively low cost method of using Masimo SET pulse oximetry to potentially diagnose congenital cardiovascular defects, with very high levels of sensitivity and specificity. The same study also showed that pulse oximeters without Masimo SET performance, fall woefully short of being useful tools for such diagnosis.
Congenital cardiovascular defects are problems with the structure or formation of the heart and major blood vessels, which occur while a fetus' heart is developing in the womb. According to the American Heart Association, congenital cardiovascular defects, also known as congenital heart disease, are the most common type of congenital malformations in newborns, present in about 1 percent of live births. Congenital cardiovascular defects range in severity with some being lethal within hours or days of birth unless diagnosed and treated. However, large numbers of these defects go undetected by routine neonatal physical examinations. In fact, 10 to 30 percent of newborn deaths from congenital cardiovascular defects in the first year are due to cases that go undetected. With advances in treatment, including the ability to perform open heart surgery on neonates as young as one day old, prognosis for newborns diagnosed with some forms of congenital cardiovascular defects is excellent, indicating that improved detection has the potential to save many lives.
In a study done by researchers from the Institute of Women's and Children's Health at the University of Gothenberg, Sweden, a new and powerful method for detecting duct dependent congenital heart defects has been described. By measuring the newborn's oxygen level in the right hand and one foot, simultaneously, with Masimo SET pulse oximetry and using specific criteria, the researchers showed that congenital cardiovascular defects could be detected with more than 90% confidence. For the study, the researchers also performed this testing with another pulse oximeter, the GE Datex Ohmeda TuffSat. However, the GE pulse oximeter obtained reliable readings in only 76% of patients. In contrast, the Masimo SET Radical pulse oximeter obtained reliable readings in 100% of the patients. Thus the researchers concluded that the non Masimo SET pulse oximeter could not be used as a screening device. The authors stated, "The data in our study clearly show that a high-performance new-generation oximeter with improved performance during low perfusion states and resistance to motion artifacts is the key to enable screening with both a high sensitivity and a low false positive rate."
Michael Liske, MD, Pediatric Cardiologist at Vanderbilt University stated, "This exciting technology has the potential for screening asymptomatic newborn infants for critical congenital heart defects. Other technologies have been limited by their sensitivity, perhaps related to errors related to patient motion, the very issue that Masimo SET addresses. The Masimo SET pulse oximeter would be the ideal tool for use in a large scale study to determine if universal screening should be implemented."
About
Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through-Motion
and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 100 clinical studies
have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry,
which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.Masimo.com.
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
Welch Allyn Expands Patient Monitor Capabilities with Masimo Pulse Oximetry Technology
Vital Signs Monitor 300 Series latest Welch Allyn monitor to offer Masimo SET SpO2
Beaverton, Ore., and Irvine, Calif., February 9, 2006 - In an effort to continue to meet the challenges caregivers in multiple environments face every day, Welch Allyn, a leading manufacturer of frontline medical products and solutions, announced the availability of Masimo SET® pulse oximetry (SpO2) in the Vital Signs Monitor 300 Series automated vital signs monitor. The offering marks the latest collaboration between the two companies in a partnership that applies Masimo pulse oximetry technology across a range of Welch Allyn patient monitoring solutions.
"We strive to offer frontline caregivers solutions that maximize their flexibility without changing the way they work," said Doug Linquest, Welch Allyn executive vice president, acute care and Asia. "Adding the option of Masimo SET SpO2 to the Vital Signs Monitor 300 Series partners the gold standard pulse oximetry technology with one of the most simple, versatile and affordable vital signs monitors out there today."
The Vital Signs Monitor 300 Series is a fully-featured patient monitor that provides simple, automated vital signs measurement before, during, and after medical procedures.
It allows caregivers to either spot-check or continuously monitor patients' blood pressure, temperature, heart rate and pulse oximetry from a single device.
"The Vital Signs Monitor 300 Series is a great product and with it Welch Allyn has satisfied a previously unmet need," said Joe Kiani, Masimo Chief Executive Officer. "We're delighted to have the opportunity to expand our partnership with Welch Allyn, and extend clinician and patient access to Masimo pulse oximetry farther than ever before."
Masimo SET (Signal Extraction Technology) pulse oximetry is a highly advanced method of monitoring the oxygenation of a patient's blood. Masimo technology has been clinically proven to read through motion and low perfusion at a high level of accuracy, with certain studies showing improved patient outcomes.
Welch Allyn offers Masimo SET pulse oximetry in a variety of other products, including the new Spot Vital Signs LXi spot-check device, Propaq CS and Propaq Encore continuous vital signs monitors and the Micropaq wireless telemetry monitor.
"As the trend continues away from hospital-based inpatient procedures to more outpatient procedures and more acute procedures, caregivers need flexible continuous monitoring solutions," Linquest said. "Masimo technology makes the Vital Signs Monitor 300 Series better equipped to meet that need."
About Welch Allyn
Welch Allyn, Inc. was founded in 1915 and is today a leading manufacturer of innovative medical diagnostic
and therapeutic devices, cardiac defibrillators, patient monitoring systems, and miniature precision lamps. Headquartered in Skaneateles Falls, New York, USA, Welch Allyn employs more than 2,100 people
and has numerous manufacturing, sales, and distribution facilities located throughout the world. Additional information on Welch Allyn and its products may be found at www.welchallyn.com.
About
Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through-Motion
and Low Perfusion pulse oximetry, Signal Extraction Technology and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 100 clinical
studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations where competing pulse oximetry devices fail. Masimo
provides its pulse oximetry technology through standalone and handheld monitors and as a monitoring parameter integrated into patient monitors from leading manufacturers, worldwide. Building
on the SET platform, in 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which uses eight wavelengths of light and breakthrough signal processing technology to noninvasively, continuously
monitor the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Additional clinically important applications of
Rainbow SET are in advanced stages of development.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications."
SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Masimo Signs Three-Year, Dual-Source Agreement with Novation for Pulse Oximetry, and New Masimo Rainbow SET™ Technology
Novation the first GPO to contract for Masimo Rainbow SET Pulse CO-Oximetry
IRVINE, California - February 2, 2006 Masimo today announced that it has signed a three-year, dual-source agreement with Novation, the health care contracting services company of VHA Inc. and the University Health System Consortium (UHC). The agreement covers Masimo SET® pulse oximetry and Masimo Rainbow SET Pulse CO-Oximetry™, including standalone monitoring devices, handhelds and sensors. The competitive bid process involved an extensive clinical review and a technology value analysis by Novation's member-based pulse oximetry task force.
Masimo is the inventor of read-through motion and low-perfusion pulse oximetry, a technology called Masimo SET, which has been proven more accurate and reliable in the most challenging clinical settings by over 100 independent clinical studies. Building on this technology platform, Masimo has recently introduced Masimo Rainbow SET, a new technology that uses eight wavelengths of light to allow clinicians to capture and monitor an unprecedented array of patient physiological data noninvasively. Rainbow SET capabilities will be available in Masimo monitors and in multi-parameter patient monitors produced by leading manufacturers.
The Rad-57™ Pulse CO-Oximeter™, the first FDA cleared Rainbow SET product from Masimo, is a handheld device that allows clinicians to detect and monitor carbon monoxide levels in the bloodstream non-invasively. In clinical studies and in the field, Rad-57 is already proving itself effective in detecting carbon monoxide poisoning in seconds, allowing accurate diagnosis and early treatment of a life-threatening problem that is frequently misdiagnosed as flu or migraine. Masimo has announced that it has other new Rainbow SET monitoring capabilities in advanced development and that the ability to noninvasively detect and monitor methemoglobin levels in the blood is pending FDA clearance. A recent Johns Hopkins study found that methemoglobinemia, a potentially lethal condition that starves the tissues of oxygen, is much more common in hospitalized patients than previously realized.
"We are happy to announce this new agreement with Novation," said Joe E. Kiani, Masimo's Chairman and CEO. "Since being added to the Novation contract in 2003, our annualized sales to Novation hospitals has increased by over 5000%, signifying the demand for our technology. Through this agreement, VHA and UHC members have access to superior Masimo SET pulse oximetry products and Rainbow SET technology that will allow earlier detection and treatment of an expanding number of potentially life-threatening conditions."
"Novation is committed to providing the best purchasing options to VHA and UHC members," said LeAnn Born, vice president of contract and program services for Novation. "This agreement demonstrates how Novation helps members purchase the latest technologies at lower costs. "
About Novation
Based in Irving, Texas, Novation was established in January 1998 through a combination of the supply programs of VHA and UHC, two national health care alliances.
These organizations used Novation contracts to purchase more than $23 billion in supplies in 2004.
About Masimo
Masimo develops innovative monitoring technologies that significantly
improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through-Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false
alarms and increased pulse oximetry's ability to detect life-threatening events. Over 100 clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood
oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide
and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.Masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact:
Brad Langdale
949-297-7009
blangdale@masimo.com
New Study Finds that Carbon Monoxide (CO) Poisoning Can Cause Myocardial Injury and Significantly Increased Long-term Mortality
New Masimo technology noninvasively measures CO levels in arterial blood in seconds, preventing delays in diagnosis and treatment
Irvine, California, February 1, 2006 - Masimo, the inventor and leader of read-though motion and low perfusion pulse oximetry and Pulse CO-Oximetry™ announced an important new study published in The Journal of American Medical Association (JAMA®) by researchers from the Minneapolis Heart Institute Foundation, which demonstrated the acute effects on myocardial tissue and resulting detrimental long term effects on patient outcomes due to moderate and severe carbon monoxide (CO) poisoning.
Dr. Timothy Henry and a team of researchers studied patients who were treated for moderate to severe CO poisoning. The study, entitled "Myocardial Injury and Long-term Mortality Following Moderate to Severe Carbon Monoxide Poisoning", found that many patients who were exposed to CO suffered myocardial injury and were three times more likely to die during the follow-up period compared to age and sex specific US mortality rates (mean follow-up period was 7.6 years).
Although moderate and severe CO poisoning is commonly associated with transient or persistent loss of consciousness, brain injury, and other severe neurological symptoms, only myocardial injury and age were found to be significant predictors of mortality in these patients. All the patients in the study received hyperbaric oxygen therapy and those patients who showed ischemia on electrocardiogram or biomarkers for myocardial injury also received cardiovascular medications. It is therefore unclear if therapeutic intervention can affect the long-term outcomes of patients who suffer myocardial injury due to CO poisoning. Nevertheless, the findings of this study contribute to the body of evidence suggesting that healthcare providers should screen all patients potentially exposed to CO for CO poisoning so that the damage due to CO poisoning can be minimized as much as possible by proper intervention.
"This study underscores the importance of immediate assessment of patients that have been exposed to carbon monoxide," said Joe Kiani, Chief Executive Officer of Masimo. "Before the introduction of Masimo Rainbow SET™ Pulse CO-Oximetry, the only way for measuring carbon monoxide in the bloodstream was a CO-oximetry test, which required a painful blood draw and potentially significant delays in diagnosis. And even though CO poisoning is the most common type of accidental poisoning in adults in the US, many hospitals do not even have invasive CO-oximeters on site. With the introduction of the Masimo Rainbow SET Pulse CO-Oximetry, hospital clinicians and first responders now have a noninvasive portable monitor that can be used anywhere to quickly diagnose and monitor carbon monoxide poisoning painlessly."
Maribeth Sayre, MD, Director of Medical Affairs of Masimo added, "CO poisoning is not only potentially fatal, but for those who survive it, treatment must be instituted immediately if long term neurological damage, and perhaps myocardial damage, is to be avoided. Dr. Henry's research is providing very important information to the medical community. A common misconception regarding CO poisoning is that it can be prevented and effectively dealt with by installing airborne CO detectors. While these devices are critical to protecting people against being overcome by extreme CO levels, they may not detect lower levels of CO. The problem is that even small amounts of CO accumulate in the blood very quickly and dissipate very slowly, so long-term or repeated exposure to low levels of CO can lead to toxic levels accumulated in the blood. Anyone with classic CO poisoning symptoms of dizziness, headaches, shortness of breath, fatigue and nausea should be screened for CO poisoning. Now with Rainbow SET, CO detection and monitoring can be done quickly and painlessly."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care- helping solve "unsolvable" problems. In 1995, the company debuted Read-Through-Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Over 100 clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. In 2005 Masimo introduced Rainbow SET and with it, Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions.
Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.Masimo.com
Masimo, SET, Signal Extraction Technology and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow and Pulse CO-Oximetry are trademarks of Masimo Labs.
Contact:
Brad
Langdale
949-297-7009
blangdale@masimo.com
Masimo and Nellcor Announce Settlement of Patent Litigation
IRVINE, California - January 23, 2006, Masimo today announced a settlement of all existing patent litigation with Nellcor, a division of Tyco Healthcare. Under the terms of the settlement, all pending patent litigation will be dismissed, and Nellcor has paid Masimo $265 million for damages through January 31, 2006. In addition, Nellcor made an advance royalty payment of $65 million related to sales of Nellcor's new products during the remainder of calendar 2006. After January 31, 2006, Nellcor will no longer ship its current 05 pulse oximetry platform, but it will continue to provide service and sensors for previously sold products. Masimo has granted Nellcor the right to sell Nellcor's new line of pulse oximetry products in exchange for an ongoing royalty.
About Masimo
Masimo, the inventor of Pulse CO-Oximetry™ and Read-Through Motion and
Low Perfusion Pulse Oximetry, was founded in 1989. Masimo's monitoring systems owe both their superior accuracy and reliability to the company's unique SET® (Signal Extraction Technology),
a solution the company invented and introduced worldwide in 1998 to address problems that have traditionally beset conventional pulse oximetry systems. Masimo SET allows accurate monitoring despite patient
motion, bright lights, signals from other electrical equipment, and low blood flow. These events and conditions, common in adult, pediatric and neonatal patient care, can cause inaccurate readings or
loss of readings with less advanced monitoring technology. Over 100 clinical studies support the conclusion that Masimo SET is the most effective pulse oximeter in the world.
Masimo Rainbow SET™ technology ushers in a new era of non-invasive monitoring, where patients at risk for respiratory and cardiac complications and those suffering from carbon monoxide and methemoglobin poisoning and other life-threatening conditions can be quickly diagnosed and treated.
Contact: Brad Langdale 949-297-7009
Award-Winning Human Volunteer Study Says New Masimo Rainbow SET™ Technology Represents a "Major Advance" in Patient Monitoring
Scene out of Star Trek: Handheld device detects carbon monoxide poisoning noninvasively, using light waves
IRVINE CA—January 20, 2006 A new study of the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter™, conducted by Steven J. Barker, PhD, MD of the University of Arizona, has received the 2006 Application of Technology award from the Society for Technology in Anesthesia.
The winning study, "New Pulse Oximeter Measures Carboxyhemoglobin Levels in Human Volunteers" evaluated the ability of the Rad-57 to directly measure the effects of carbon monoxide inhalation in humans. The lethal effects of carbon monoxide are caused by the conversion of normal hemoglobin in the blood to an abnormal form called carboxyhemoglobin, or "COHb." Levels of COHb higher than 50% are potentially fatal, and caused the recent deaths of the coal miners in West Virginia. This study compared the COHb readings from the Rad-57 with blood sample measurements made by CO-Oximeters, which are currently used to determine COHb levels in hospital laboratories. The CO-Oximeter is a large, expensive machine that requires a blood sample, whereas the Rad-57 is small, portable, and measures COHb with a simple finger-clip sensor. The Arizona researchers found that the Rad-57™ performed within its specifications, accurately measuring the changing COHb levels in the volunteers' blood. The study concluded that this new technology represents a major advance in the monitoring of oxygenation. This is the second time technology pioneered by Masimo is being honored by the STA with such an award.
Unmasking
a silent killer—carbon monoxide—with light waves
"We believe that this device represents a major advance in patient monitoring," said Dr. Barker,
the lead author and Head of the Department
of Anesthesiology at University of Arizona. "Carbon monoxide poisoning can be a life-threatening problem in the Operating Room, as well as in many settings
outside of the hospital. This new technology
allows diagnosis in seconds, even in field conditions by first responders. By allowing earlier diagnosis and treatment, this will have a significant effect
on patient care."
"We are delighted
to hear of the award given to the University of Arizona research team," said Joe E. Kiani, Masimo Chairman and CEO. "Ten years ago, Dr. Barker and Masimo won
a similar STA award for Masimo SET®, or Signal
Extraction Technology. At that time, our achievement was to monitor oxygen saturation levels accurately for the first time during conditions such as patient
motion and low perfusion. Now, building on
SET, we are able to monitor carboxyhemoglobin and methemoglobin levels, and soon, we hope, additional vital physiologic parameters, noninvasively. This is
history repeating itself in the most encouraging
way."
"We are especially pleased that Dr. Barker and his colleagues on the University of Arizona research team have received this recognition," Kiani added. "Dr. Barker is the author of over 150 scholarly works, and one of the leading researchers in oxygen monitoring. To provide Masimo with the scientific advice we need and a strong connection to the medical community, we have invited Dr. Barker to work with us as the Chairman of our Scientific Advisory Board and as a member of the Masimo Board of Directors. I am delighted to say that he has accepted, and we will be able to lead Masimo better with his guidance."
About Masimo
Masimo, the inventor of Pulse CO-Oximetry™ and Read-Through Motion and Low Perfusion Pulse Oximetry, was founded in 1989. Masimo's monitoring systems owe both their superior accuracy and reliability to the company's unique SET (Signal Extraction Technology), a solution the company invented and introduced worldwide in 1998 to address problems that have traditionally beset traditional pulse oximetry systems. Masimo SET allows accurate monitoring despite patient motion, bright lights, signals from other electrical equipment, and low blood flow. These events and conditions, common in adult, pediatric and neonatal patient care, can cause inaccurate readings or loss of readings with less advanced monitoring technology. Over 100 clinical studies support the conclusion that Masimo SET is the most effective pulse oximeter in the world.
Masimo's Rainbow™ technology ushers in a new era of non-invasive monitoring, where patients at risk for respiratory and cardiac complications and those suffering from carbon monoxide poisoning and other life-threatening conditions can be quickly diagnosed and treated.
Carbon monoxide poisoning: visible tragedies, an invisible epidemic
Two weeks ago, the tragic news of the 12 West Virginia coals miners who died as a result of carbon monoxide (CO) poisoning, reminded us what a lethal threat is posed by this gas, the most frequent cause of accidental poisoning. Every winter, local papers carry stories of entire families fatally poisoned at home by malfunctioning furnaces or space heaters.
Subacute, chronic CO poisoning is less visible, but may be much more common. In many cases, victims are unaware that they are being poisoned and may easily be misdiagnosed or never examined by health care professionals. With the March 2005 introduction of the Rad-57™ Rainbow SET Pulse CO-Oximeter™, which allows noninvasive diagnosis of carbon monoxide levels in the community as well as in the Emergency Department, Masimo has received field reports of carbon monoxide poisoning diagnosed in numerous people who were asymptomatic or only mildly symptomatic.

A toxic firehouse
In November, a Masimo representative was conducting a Rad-57 inservice for a group of Midwestern firefighters whose Department had purchased units to use in firefighter rehabilitation. CO readings were normal for the fire chief and several other firefighters attending the inservice, but were elevated with three individuals who were stationed at the same firehouse. None of the men smoked. The group's first reaction was to question the accuracy of the Rad-57 readings, but when questioned, all three men admitted that they had been feeling slightly ill in recent weeks. Later, it was discovered that a hose installed to vent tail pipe exhaust from the fire engine was defective. Fire engines are often kept running in the fire apparatus bays before calls, one floor beneath the firefighters' living and working areas.
The poisoned firefighters were well acquainted with the dangers of CO poisoning, trained to protect themselves from inhaling the gas during fires, but they never associated the mild flu-like symptoms they were experiencing with carbon monoxide poisoning, nor had they mentioned their symptoms to others. "It's the macho tradition," one said.
After the venting system was repaired, the men continued to be monitored with the Rad-57—their CO readings returned to normal.
A
potentially deadly commute
In December, an EMS team in a Rocky Mountain town made a similar "save". They were conducting an inservice on their newly acquired Rad-57 unit to a number of public service employees, demonstrating how smokers routinely recorded elevated CO levels. One attendee, a non-smoker, showed markedly elevated levels of carboxyhemoglobin. Alarmed, the EMS team contacted the local Fire Department, which tested the woman's house for carbon monoxide gas—with negative results.
Investigating further, the Fire Department tested the woman's car with the engine running and found that the passenger compartment was heavily contaminated by carbon monoxide—the exhaust system was defective. Winter temperatures in the mountains were below zero many days, and the poisoned woman was in the habit of warming her car up for ten minutes when she commuted back and forth to work. During every morning and evening trip she was exposed to high levels of carbon monoxide, at risk of a lapse in consciousness while driving on icy mountain roads.
Like the firemen, the poisoned woman had been feeling "not quite right" lately, but had decided that she was just coming down with a cold.
Unmasking the Great Imitator
For clinicians, diagnosing carbon monoxide poisoning from symptoms can be extremely difficult. Symptoms reported by poisoned patients are often identical to those reported by people with colds, flu, migraine, food poisoning and a host of other ailments.
Consequently, the National Academy of Clinical Biochemistry recommends that clinicians routinely assess the percentage of blood carboxyhemoglobin by cooximetry to screen patients with flu-like symptoms in the Emergency Department, particularly in communities where combustion is used for heating during the heating season. Co-oximetry requires a blood sample be taken in a hospital setting and sent to the lab for analysis. With the introduction of Rad-57 Pulse CO-Oximetry, testing is painless, inexpensive and can be completed in seconds. The routine use of pulse CO-Oximetry by ED, EMS and other health care professionals can play an important role in identifying undetected carbon monoxide poisoning, helping reduce morbidity and mortality and preventing the long term neurological damage that can occur through repeated CO exposure.












